Home Gardening Fertilizing Part 1: Fertilizers 101
Home Gardening Fertilizing | Part 1: Fertilizers 101
by Jim Farr
17 Known Essential Chemical Nutrients
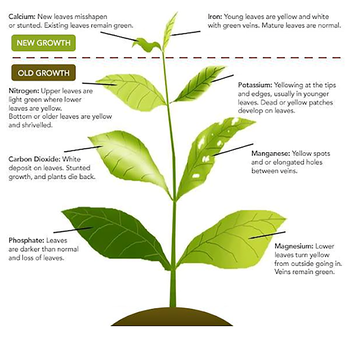
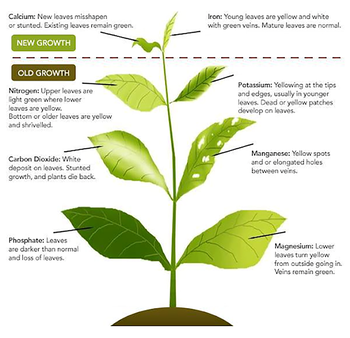
There are 17 known essential chemical nutrients or elements needed by plants for growth. Absence or insufficient levels of an essential nutrient results in abnormal growth or symptoms that are characteristic for that deficiency in plants. For example, yellowing between veins (interveinal chlorosis) in new leaves are indicative of an iron (Fe) deficiency. Yellowing of the entire leaf and lack of vigor in the plant may be an indication of low nitrogen. In most cases, the function of the essential nutrient cannot be replaced by another element or chemical compound.
What is a Fertilizer
A fertilizer is any substance that is added to the soil, sprayed on the aerial part of plants, or applied as a coating on seeds to supply one or more plant nutrients. Plant nutrients are broadly defined as chemical elements or compounds that are necessary for the growth and reproduction of a plant. Healthy soils contain the 17 nutrients that plants need. Three of these come from air and water (carbon, oxygen and hydrogen). The remaining 14 come from minerals in the soil. Over time plants will deplete the soil of the mineral nutrients required for healthy growth and reproduction. The purpose of a fertilizer is to replenish the soil with the depleted mineral nutrients to restore the soil to a healthy condition.
Typically, fertilizers contain the most important of the mineral nutrients, nitrogen, phosphorus and potassium. These three nutrients play essential roles in the growth and reproduction of plants. These nutrients along with carbon dioxide and water function as raw material for use in the synthesis of functional and structural compounds in plants such as chlorophyll, sugars and carbohydrates.
Types of Nutrients
Essential elements are grouped into macronutrients- those elements that are present in plants in large concentrations (1 to 10 mg per gram dry matter), and micronutrients or trace elements - chemicals that are present in low concentrations in plants (less than 0.1 mg per gram of dry matter).Several macronutrients (carbon (C), hydrogen (H), and oxygen (O)) come from air and water naturally in the environment and are not added to commercial fertilizers. Carbon, Hydrogen and Oxygen make up 85-98% of the total fresh weight of a plant. When a plant is watered and it is exposed to air it receives ample amounts of these essential macronutrients.
Nitrogen (N), phosphorus (P) and potassium (K) are known as the primary macronutrients. Calcium (Ca), magnesium (Mg), and sulfur (S), are needed in lesser quantities and are considered secondary macronutrients. All together these six primary and secondary macronutrients make up between 3-12% of the fresh plant mass.
The micronutrients (less than 0.1 mg per gram of dry matter) include boron (B), chlorine (Cl), cobalt (Co), copper (Cu), iron (Fe), manganese (Mn), molybdenum (Mo), and zinc (Zn) although iron can reach secondary macronutrient levels in some plants. These make up about .5% of the fresh plant mass.
There are other recognized micronutrients that play important roles in some plants. These include nickel (Ni), sodium (Na), selenium (Se), and silicon (Si). Sodium is essential for the concentration of carbon in grasses. In other plants, excess sodium can be unhealthy. Some plants can not tolerate much sodium and can even die from too much sodium in the soil. Other plants have adapted to salt in the soil such as those near the coasts. To read more about the effects of sodium on plants and which ones are the most salt-sensitive see the blog article Using Recycled Water - What you Should Know which discusses the effect of salt on plants.
Selenium is toxic to most plants but certain plants accumulate selenium as a defense against predation. In locoweed, for example, selenium functions as a macronutrient. Silicon, because of its recognized role in plant nutrition, especially under “stress” conditions, has been referred to as a quasi nutrient. Nickel is the most recently discovered micronutrient and while not included in the 17 essential nutrients it does play a role in legumes in the nitrogen fixation process.
Reading Fertilizer Labels
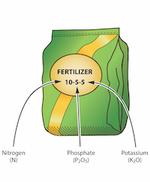
Organic fertilizers have gained in popularity in recent years. Organic fertilizers are those where the source of the nutrients was originally from a plant or animal. Some organic fertilizers provide nitrogen as a mix of amino acids and peptides, the building blocks of living organisms. The amino acids are immediately used by the plant and microbial activity is needed for the peptides to be converted into plant usable form. Soy based fertilizer provides all the nitrogen in the form of plant available amino acids.
Image 6
Fertilizers come in a variety of formulations (dry powder, granular, liquid) that can meet the needs of any grower and their crops. There are advantages and disadvantages to the different fertilizer formulations and fertilizer selection should be based on crop type, crop status, environment, and cost. Some fertilizers are formulated to be “slow release” which means that rather than release all of the nutrients all at once in the soil, they spread the release of these nutrients over a much longer period. These have certain advantages particularly in helping to prevent “fertilizer burn” where too much fertilizer released at once can harm or even kill a plant.
We have talked about what fertilizers are, what they are composed of and what different forms they come in. But the home gardener still is left with the task of figuring out when to use fertilizers and how much to use and fertilizer requirements for specific plants. These questions will be answered in part 2 and will provide the home gardener with a practical guide to using fertilizers in their gardens.
Still have questions?
You may contact the Help Desk for more information about using fertilizers in your garden or for other gardening tips and solutions.